CTD (Common Technical Document) is a set of specification for application dossier. So it is for the registration of Medicines and designed to be used across Europe, Japan and the United States. Quality, Safety and Efficacy information is assembled in a common format through CTD .Above all the CTD is maintained by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
eCTD: Firstly we understood common technical document. Secondly Its electronic version is called electronic Common Technical Document (eCTD), in other words both are same but one is simple form and other is electronic.
eCTD composed of two types of specificatios
Content Specification—-As defined by ICH
Technical Specification—-Electronic Software’s
The Common Technical Document is divided into five modules :-
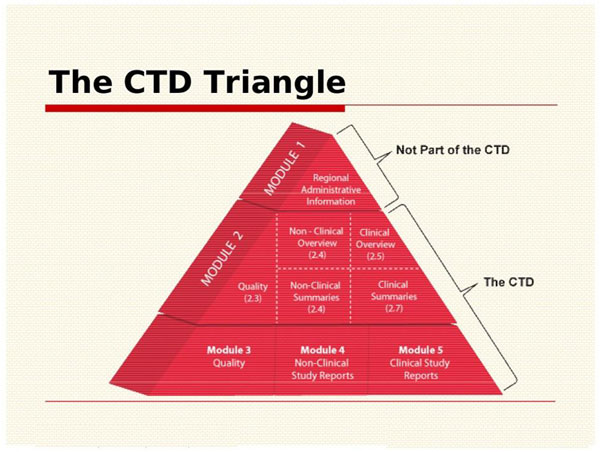
- Module 1 : Administrative information and prescribing information – Module 1 is not strictly included since it contains documents that are specific to each region. For example- application forms or the proposed label. In conclusion this module will not be discussed in any further detail in this page since the content and format of this module is specific to individual Regulatory Authorities.
- Module 2 : Common Technical Document Summaries (Overview and summary of modules 3 to 5) – Module 2 contains seven sections that should be maintained in the following order:
2.1 Table of contents
2.2 Introduction
2.3 Quality Overall Summary
2.4 Non-clinical Overview
2.5 Clinical Overview
2.6 Non-clinical Written and Tabulated Summaries
2.7 Clinical Summary. - Module 3 : Quality – Module 3 presents the chemistry, manufacturing,
and controls reports for the product included in the registration dossier. Full details of what should be included in Module 3 are provided in the ICH M4Q guideline.5 Sections on both drug substance
and drug product are included in this module. - Module 4 : Preclinical Study Reports – Module 4 presents the non-clinical reports included in the dossier. so the structure and content of Module 4 is specified in the ICH M4S guidelines
- Module 5 : Clinical Study Reports (Clinical studies) – Module 5 presents the clinical reports included in the dossier. Most importantly the structure and content of Module 5 is specified in the ICH M4E guidelines.Therefore it provide a specific placement of clinical study reports and related information . It lead to clarify to simplify preparation and review and to ensure completeness. The placement of a report is determined by the primary objective of the study. Each report appears in only one section. If there are multiple objectives, the study should be cross-referenced in the various sections.
Please have a look at our Blog
AUTHOR – SAMPRGMR
